

for use after liposuction, and the
only device that is FDA-cleared for
contracting subcutaneous tissue.


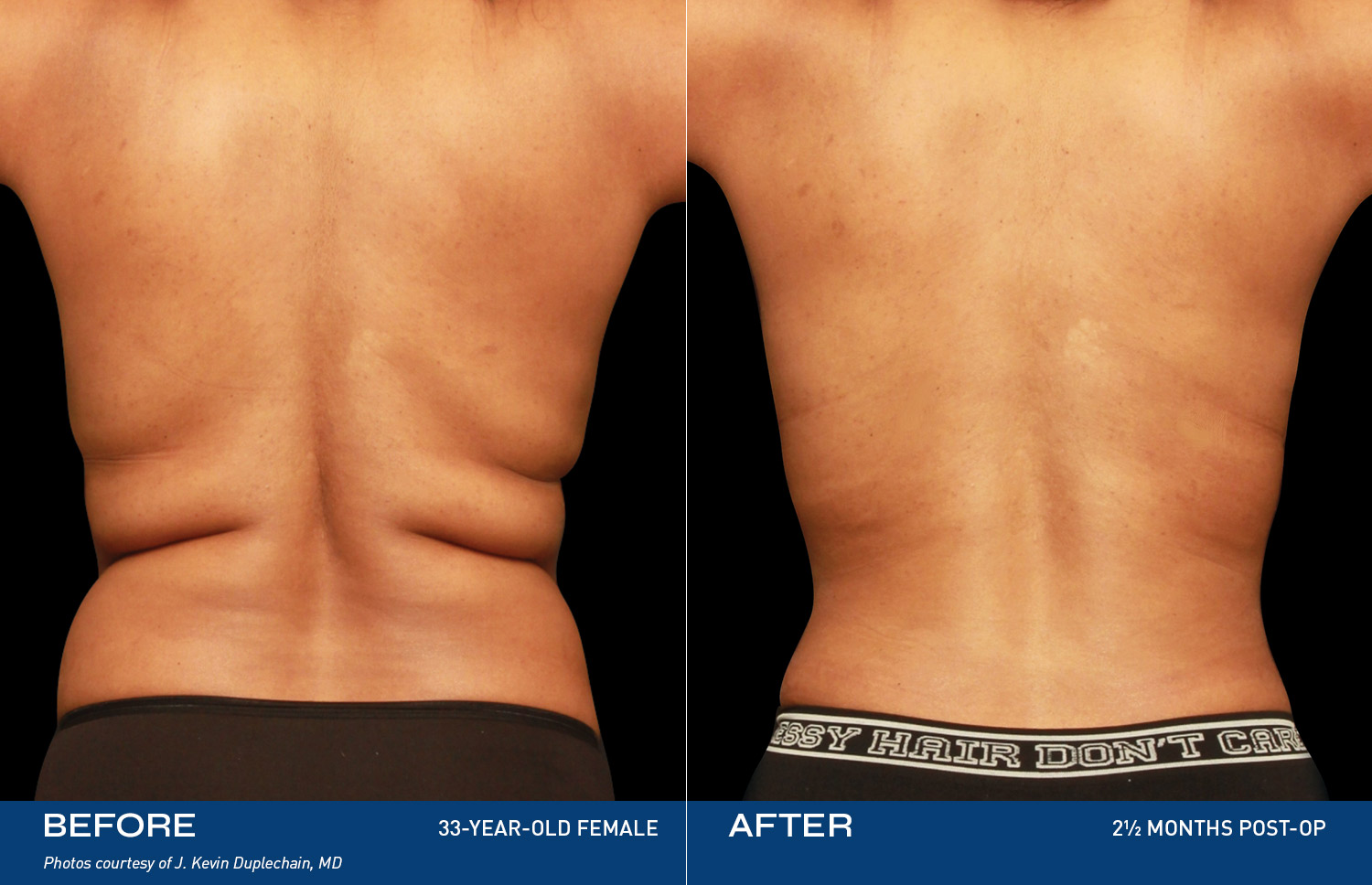
REAL RESULTS
PHYSICIAN FINDER
Enter your Name, Email and Zip Code below to find physicians near you that use Renuvion.
RESHAPING THE WAY PEOPLE LOOK AND FEEL THROUGH THE RENEWING LIVES CAMPAIGN
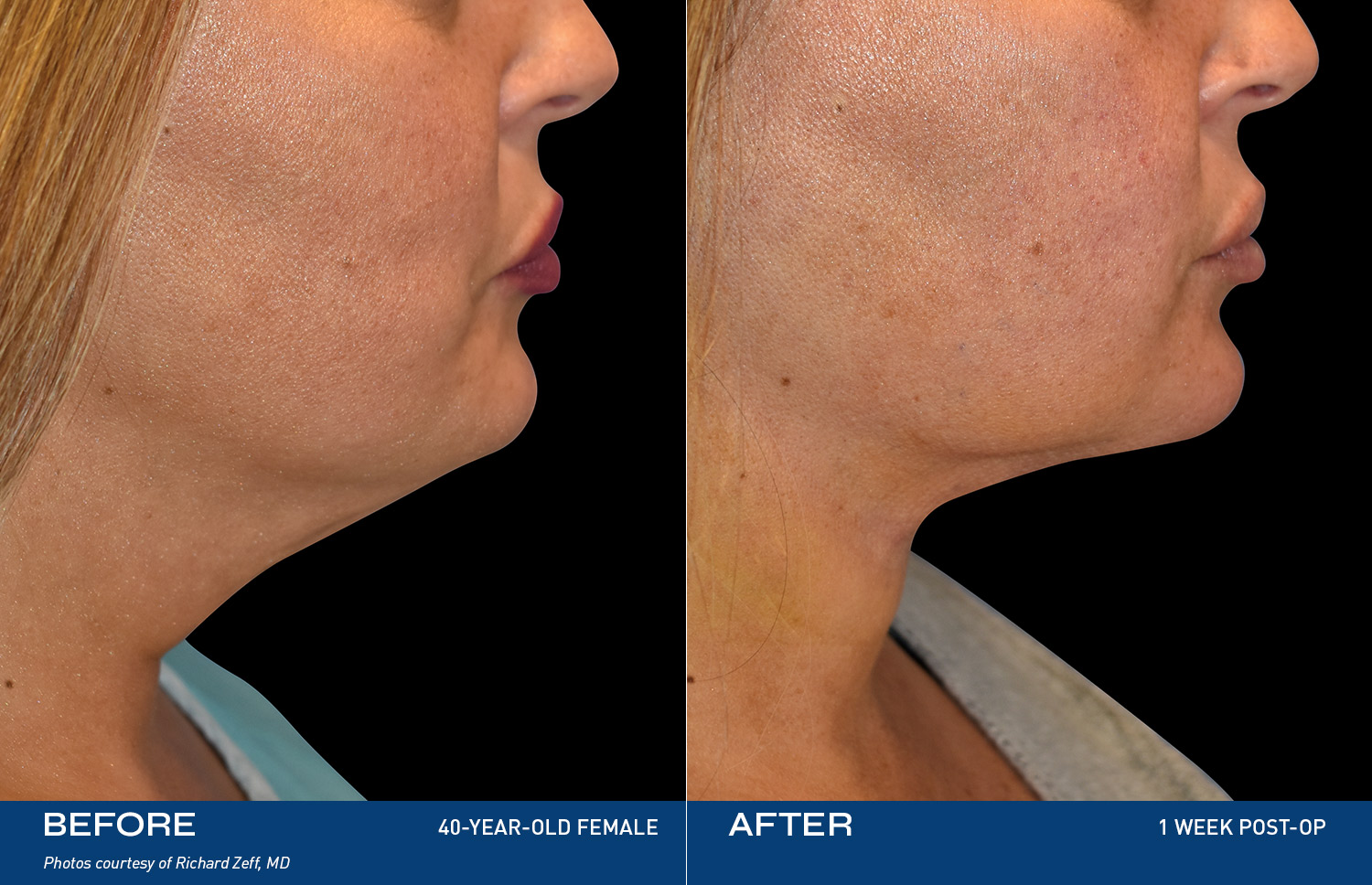
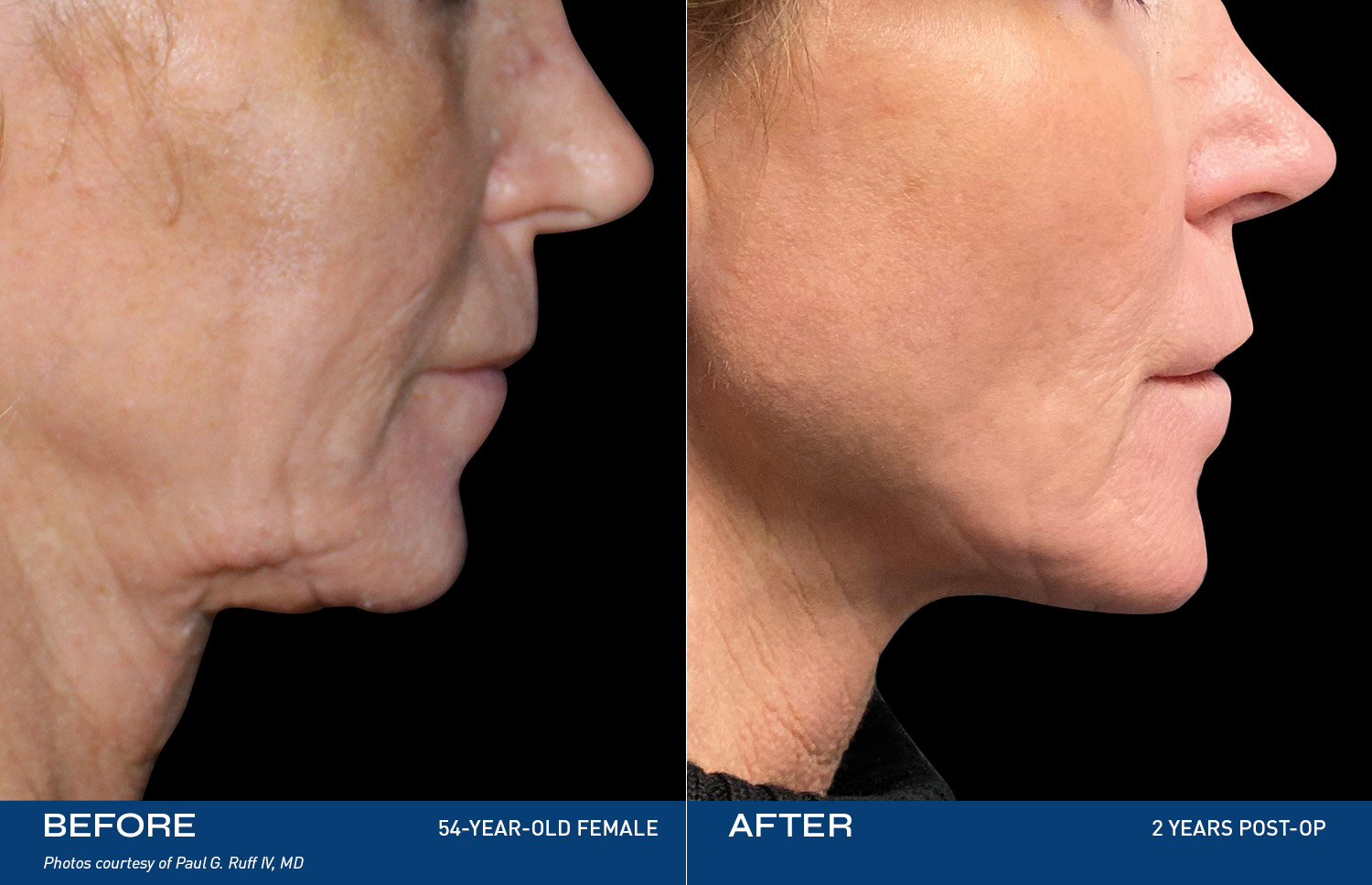
Renuvion, a safe and innovative energy-based technology, uses controlled heat to treat loose skin, contracting the collagen bands that stretch when skin may age or experience weight gain. The result: you see yourself the way you're supposed to and feel even better.
Over the last six years, aesthetic surgeons have performed more than 350,000 Renuvion treatments, providing patients with outcomes that improve their physical, mental and emotional health and appearance.
To learn more about how Renuvion works, click here.

REAL RESULTS
BEFORE & AFTERS
"I tried so many things, but my skin was still loose. After Renuvion my neck and jawline look so smooth and it feels tight like it used to. I'm so happy with the result."
- Renuvion Patient
Meet some of our participating Renuvion Surgeons

doctor
Michael Kluska
Meadows Surgical Arts
Commerce, GA
@drdaddyk

doctor
Ashley Amalfi
Quatela Center for Plastic Surgery
Rochester, NY
@drashleyamalfi

doctor
Drew Ordon
Plastic Surgery Institute
Beverly Hills, CA
@drandrewordon

doctor
David Shafer
Shafer Clinic
New York, New York
@shaferclinic
SHARE YOUR STORY
CONTOURING:
Not all indications are approved in all markets; check with your local sales representative for further information.
The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma where coagulation/contraction of soft tissue is needed. Soft tissue includes subcutaneous tissue. The Renuvion APR Handpiece is intended for the coagulation of subcutaneous soft tissues following liposuction for aesthetic body contouring. The Renuvion APR Handpiece is indicated for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region. The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma for cutting, coagulation and ablation of soft tissue during open surgical procedures. The Renuvion APR Handpiece is intended to be used with compatible electrosurgical generators owned by Apyx® Medical.
As with any aesthetic procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use. Risk associated with the use of the Renuvion APR may include: helium embolism into the surgical site due to inadvertent introduction into the venous or arterial blood supply system, unintended burns (deep or superficial), pneumothorax, temporary or permanent nerve injury, ischemia, fibrosis, infection, pain, discomfort, gas buildup resulting in temporary and transient crepitus or pain, bleeding, hematoma, seroma, subcutaneous induration, pigmentation changes, increased healing time, scarring, asymmetry and/ or unacceptable cosmetic result. Please see the instructions for use for more detailed information.
FACIAL RENEWAL:
The Renuvion Dermal Handpiece is indicated for dermatological procedures for the treatment of moderate to severe wrinkles and rhytides, limited to patients with Fitzpatrick skin types I, II, or III.
Warning: Application of more than one treatment pass in the perioral area, on the forehead, and along the jawline has been associated with hypertrophic scarring.
Risks associated with the use of the Renuvion Dermal System include but are not limited to hypertrophic scarring, milia/acne, telangiectasia (spider veins), skin discoloration/hypopigmentation, dormant infection reactivation, infection, bruising or bleeding. As with any procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use, refer to the IFU for further information.
References:
- FDA 510(k) Premarket Notifications K230272 & K223262.
- Duncan DI and Roman S. Helium Plasma Subdermal Tissue Contraction Method of Action. Biomed J Sci & Tech Res 31(2)-2020. BJSTR. MS.ID.005075.
RENEWING LIVES CAMPAIGN TERMS & CONDITIONS:
Apyx Medical Corporation (“Apyx” or “Sponsor”) is proud to announce the “Renewing Lives” Campaign (the “Campaign”), a nationwide promotional effort seeking to share the stories of individuals who can most benefit from Renuvion procedures. The primary focus of the Campaign is to enable real people to share their stories and embark with Apyx on a transformative journey of self-realization. To that end, up to 12 individuals will be selected to become the faces of this exciting new Campaign, showcasing the benefits that Apyx’ s advanced Renuvion technology can provide when paired with skilled surgical providers. The individuals selected to participate will, without charge, receive a Renuvion procedure and authentically engage with prospective patients on the results and the impact that Renuvion has had on their lives. In order to have the chance to participate in this Campaign, prospective participants must review and comply fully with the following terms and conditions of the Campaign (the “Terms and Conditions”).
NO PURCHASE NECESSARY TO ENTER OR PARTICIPATE.
You may enter the Campaign only if you are a legal resident of the 50 United States or the District of Columbia. Entries originating from any other jurisdiction are not eligible for entry. You are not authorized to participate in the Campaign if you are not located within the 50 United States or the District of Columbia. This Campaign is governed exclusively by the laws of the United States. You hereby represent and warrant that you are not represented in the modeling space. Selection to participate in the Campaign is contingent upon compliance with all requirements set forth herein.
1. How to Enter. You must click on the following link: renuvion.com/renewinglives and share a submission detailing who you are and how a Renuvion procedure could assist you (the “Submission”). Participants will be selected based upon the sole determination of Sponsor’s panel of judges, pursuant to the criteria detailed below. All Submissions and the content thereof are the sole and exclusive property of Sponsor and the receipt of a Submission will not be acknowledged or returned. You will only be eligible to participate if you submit a fully completed Submission. Limit one Submission per eligible participant. Proof of Submission will not be deemed proof of receipt by Sponsor.
2. Selection Process Criteria. All eligible Submissions will be evaluated against a set of criteria by a panel of judges selected by Sponsor. Selected entries will have demonstrated the most compelling reason to participate in the Campaign. The selection criteria includes, but is not limited to:
a. Personal Story: Submit a compelling personal story explaining the need for the Renuvion procedure, highlighting struggles with body image and the impact of loose skin on physical and emotional well-being, and any extenuating circumstances.
b. Body Area: Specify the body area(s) to be treated (e.g., abdomen, thighs, arms, neck) with visible signs of loose skin or sagging.
c. Medical Eligibility: Undergo evaluation and approval by a participating surgeon, providing a comprehensive medical history and any relevant medical records.
d. Commitment to Process: Agree to travel at own expense for consultations and follow-up appointments, adhering to all pre-operative and post-operative guidelines set by the surgeon.
e. Influencer Agreement: Enter into an Influencer Agreement with the Sponsor, sharing the experience throughout the campaign, including before-and-after photos and updates on social media, and executing an Affidavit of Eligibility, Publicity Release, and Liability Waiver.
3. Start/End Dates. Campaign begins at 12:00 a.m. Eastern Time (“ET”) on June 25, 2024 and ends at 12:00 a.m. (ET) on December 31, 2024. All Submissions must be completed by December 31, 2024.
4. Eligibility. Participation is open only to legal residents of the 50 United States or the District of Columbia, who are 21 years of age or older as of date of entry. Void outside of the 50 United States and the District of Columbia, and where prohibited, taxed or restricted by law. Employees, officers and directors of Sponsor and its subsidiaries, affiliates, partners, dealers, advertising and promotions agencies, manufacturers or distributors of Campaign materials and their immediate families (parents, children, siblings, spouse) or members of the same household (whether related or not) of such employees, officers, or directors are not eligible to enter. Campaign may only be entered in or from the 50 United States or the District of Columbia, and entries originating from any other jurisdiction are not eligible for entry. All federal, state and local laws and regulations apply. All decisions by Sponsor are final in all aspects of the Campaign.
In connection with the Campaign, selected participants must:
• Be prepared to travel at their own expense to the office of a participating surgeon and satisfy all requirements for treatment after completing a complimentary consultation.
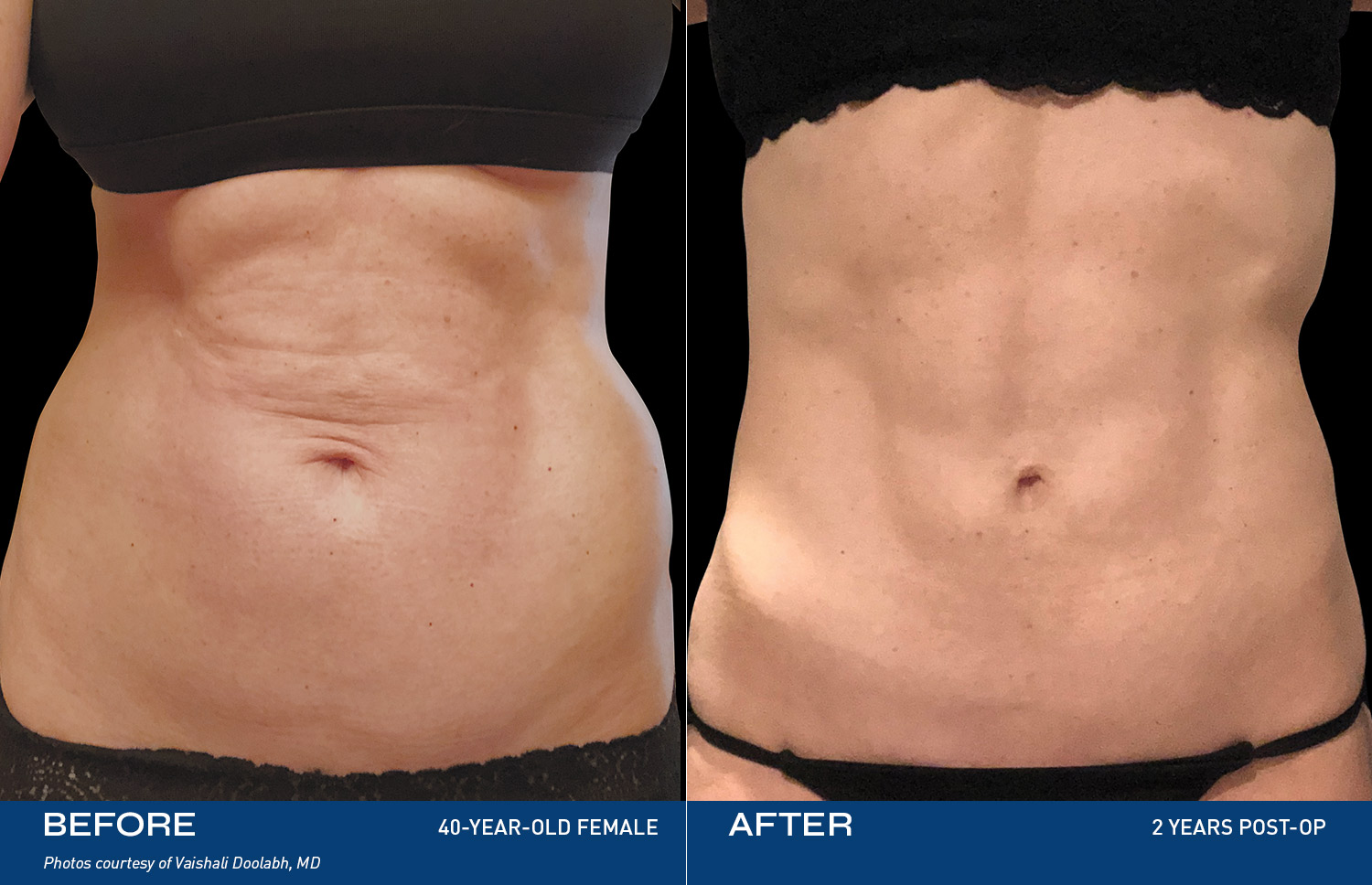
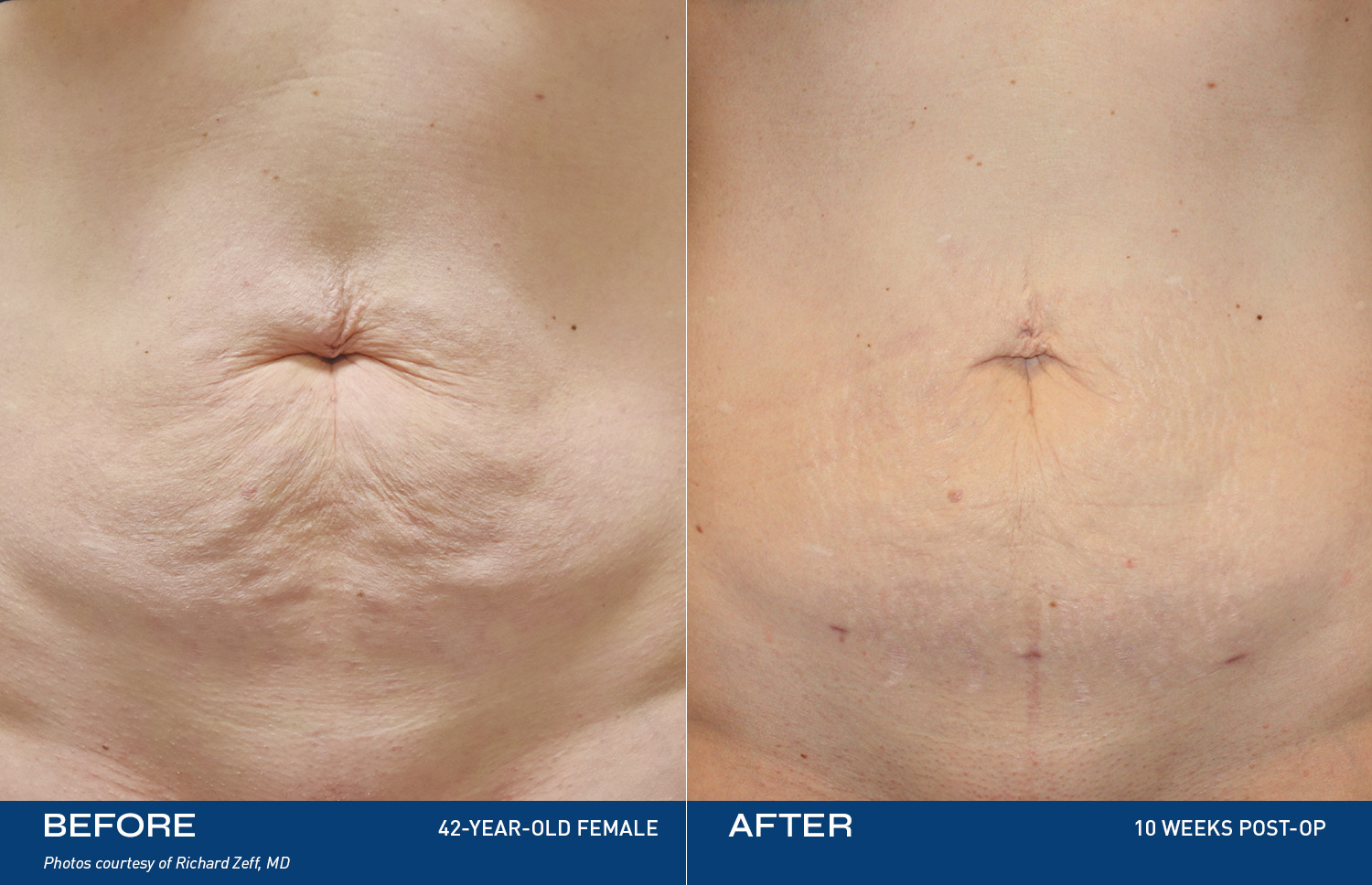
• Be eligible and approved for the Renuvion procedure by the participating surgeon, adhering to all pre-operative and post-operative guidelines set forth by the participating surgeon. The Renuvion procedure uses controlled heat to treat loose skin by contracting the collagen bands that stretch due to aging or weight gain. In just minutes, this direct and precise targeting creates a contouring effect. Body contouring, or body sculpting, refers to surgical procedures that improve the appearance of skin and tissue after weight loss. This procedure may involve getting rid of extra skin, eliminating excess fat, and reshaping or contouring the area. Surgical body contouring following weight loss addresses excess sagging skin and fat while improving the shape of the underlying support tissue, resulting in a better-proportioned appearance with smoother contours. The wand is inserted under the skin and delivers a unique combination of radiofrequency energy and helium plasma to the collagen-rich tissue, contracting these collagen fibers and pulling the skin closer to the muscle for a more contoured appearance.
• Agree not to apply or seek additional reimbursement or combined benefit from any third party insurance (including Medicaid, Medicare, Tricare, managed care or any private insurance).
• Execute an Affidavit of Eligibility, Publicity Release, and Liability Waiver.
• Enter into an Influencer Agreement with Sponsor, detailing his/her role throughout the Campaign.
5. Selection/Notification; Redemption. Participants who are selected by Sponsor to participate in the Campaign will receive the following: (i) a free Renuvion consultation, (ii) a free Renuvion procedure from a participating plastic surgeon to one specific region of the participant’s body, and (iii) an after-care package (each detailed below). Selected participants will be notified via email. Selected participants are limited to one Renuvion procedure and after-care package per person.
Selected participants must redeem and complete the free consultation with a participating plastic surgeon within 60 days of receiving notice that they have been chosen to participate in the Campaign. Notwithstanding Sponsor’s initial selection of a participant, each selected participant’s ability to receive a Renuvion procedure will be determined by the surgeon who conducts the consultation.
Any unused services or products forming a part of the after-care package are not transferable or exchangeable. Services and products that comprise an after-care package may include (but are not limited to) compression garments, follow-up consultations, prescription medications, and specialized skincare .
In the event a selected participant fails to timely redeem and complete his or her free consultation or it is determined by the surgeon performing the free consultation that the selected participant is unable to undergo the desired Renuvion procedure (or an applicable alternative Renuvion procedure), the Renuvion procedure shall be forfeited and the selected participant shall be removed from future participation in the Campaign. Participants who do not comply with these Terms and Conditions or attempt to interfere with the Campaign in any way shall be disqualified. Sponsor is not responsible if the Campaign cannot take place or if any Renuvion procedure or any related after-care services cannot be performed or products cannot be delivered due to travel cancellations, medical conditions, delays or interruptions due to acts of God, acts of war, natural disasters, pandemics, labor disputes, weather or acts of terrorism.
6. Persona and Rights of Publicity and Marketing. Providing a Submission constitutes participant’s consent to give Sponsor a royalty-free, irrevocable, perpetual, non-exclusive license to use, reproduce, modify, publish, create derivative works from, and display such Submissions in whole or in part, on a worldwide basis, in perpetuity, and to incorporate it into other works, in any form, media or technology now known or later developed, for any purpose whatsoever, including for promotional or marketing purposes free of remuneration. Participant consents to Sponsor sharing content related to participant’s respective Submission, including but not limited to his or her information, story and photographs, with global partners of Sponsor. If selected, the participant agrees to participate in any marketing and public relations activities relating to the Campaign as may be requested by Sponsor, including, but not limited to participation in promotional material, media promotions, interviews and appearances. In addition to these Terms and Conditions, selected participants are required to sign a release form and influencer agreement prior to their consultation with a participating surgeon to declare that they are willing to participate in marketing and public relations relating to the Campaign. Participants may be required to make reasonable travel arrangements and to be available during business hours. Sponsor may, but is not required, to reimburse a participant for the time or travel associated with meeting these publicity and marketing requirements.
7. Release; Limitation of Liability. BY PROVIDING A SUBMISSION, PARTICIPANTS AGREE TO RELEASE AND HOLD HARMLESS SPONSOR AND ITS ADVERTISING AND PROMOTIONS AGENCIES AND THEIR RESPECTIVE PARENT COMPANIES, SUBSIDIARIES, AFFILIATES, PARTNERS, REPRESENTATIVES, AGENTS, SUCCESSORS, ASSIGNS, EMPLOYEES, OFFICERS AND DIRECTORS (COLLECTIVELY, “RELEASEES”), FROM ANY AND ALL LIABILITY, FOR LOSS, HARM, DAMAGE, INJURY, COST OR EXPENSE WHATSOEVER INCLUDING WITHOUT LIMITATION, PROPERTY DAMAGE, PERSONAL INJURY AND/OR DEATH WHICH MAY OCCUR IN CONNECTION WITH, PREPARATION FOR, TRAVEL TO, OR PARTICIPATION IN THE CAMPAIGN, OR POSSESSION, ACCEPTANCE AND/OR USE OR MISUSE OF ANY SERVICES OR PRODUCTS THAT ARE RECEIVED IN CONNECTION WITH PARTICIPATION IN ANY CAMPAIGN-RELATED ACTIVITY AND FOR ANY CLAIMS BASED ON PUBLICITY RIGHTS, DEFAMATION, INVASION OF PRIVACY, COPYRIGHT INFRINGEMENT, TRADEMARK INFRINGEMENT OR ANY OTHER CAUSE OF ACTION. IN NO EVENT WILL THE RELEASEES BE RESPONSIBLE OR LIABLE FOR ANY DAMAGES OR LOSSES OF ANY KIND, INCLUDING DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES ARISING OUT OF OR RELATED IN ANY WAY TO THE CAMPAIGN. WITHOUT LIMITING THE FOREGOING, THE CAMPAIGN AND ALL AFTER-CARE PRODUCTS ARE PROVIDED “AS IS” WITHOUT WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO, THE IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NON-INFRINGEMENT. SOME JURISDICTIONS MAY NOT ALLOW THE LIMITATIONS OR EXCLUSION OF LIABILITY FOR INCIDENTAL OR CONSEQUENTIAL DAMAGES OR EXCLUSION OF IMPLIED WARRANTIES SO SOME OF THE ABOVE LIMITATIONS OR EXCLUSIONS MAY NOT APPLY TO YOU. CHECK YOUR LOCAL LAWS FOR ANY RESTRICTIONS OR LIMITATIONS REGARDING THESE LIMITATIONS OR EXCLUSIONS.
8. Personal Data. Personal information is being collected in order to administer the Campaign. By creating and providing a Submission, the participant acknowledges that a further primary purpose for collection of such personal information by the Sponsor is to enable the Sponsor to use the information to assist the Sponsor in improving its goods and services and to contact the participant. The Sponsor may share information with any related entities, contractors, agents or suppliers of the Sponsor, who may also contact the participant with special offers in the manner set out in this provision. By providing a Submission, the participant acknowledges and agrees that the Sponsor may use the participant’s personal information in the manner set out in these Terms and Conditions. Sponsor will be collecting personal information, in accordance with its privacy policy. Please review Sponsor’s privacy policy at https://www.renuvion.com/privacypolicy/. By creating and providing a Submission, participants hereby agree to Sponsor’s collection and usage of their personal information and acknowledge that they have read and accepted Sponsor’s privacy policy.
9. Additional Terms and Conditions. Apyx reserves the right to terminate the Campaign at any time for any reason. Participants agree that the Terms and Conditions and the Campaign will be subject to and construed under the laws of the State of Florida without regard to its conflict of laws principles. The parties waive all rights to trial in any action or proceeding instituted in connection with these Terms and Conditions and/or the Campaign. Any dispute, controversy, or claim arising out of or relating to these Terms and Conditions and/or the Campaign shall be settled by binding arbitration in accordance with the commercial arbitration rules of the American Arbitration Association. Any such controversy or claim shall be arbitrated on an individual basis, and shall not be consolidated in any arbitration with any claim or controversy of any other party. The arbitration shall be conducted in the State of Florida, City of Tampa. Notwithstanding the foregoing, the parties irrevocably submit and consent to the exclusive jurisdiction and venue of the state and federal courts located in or closest to Hillsborough County in the State of Florida for any matters in connection with the entering of any judgment on an arbitration award in connection with these Terms and Conditions and/or the Campaign, and/or to seek injunctive relief to enforce the obligations of the other under these Terms and Conditions and/or the Campaign. The parties agree not to raise the defense of forum non conveniens. By creating and providing a Submission, participant fully and unconditionally agrees to these Terms and Conditions, which are final and binding in all matters related to the Campaign.
Campaign: The Renewing Lives Campaign.
Name of Sponsor: Apyx Medical Corporation, located at 5115 Ulmerton Road, Clearwater, FL 33760-4004.
Description of Renuvion Procedures: Renuvion is inserted under the skin and delivers a unique combination of radiofrequency energy and helium plasma to the collagen-rich tissue that’s a primary cause of loose skin. This energy contracts these collagen fibers and pulls the skin down closer to the muscle, for a more contoured appearance. Renuvion can be performed under both general and local anesthesia. It is always best to discuss these treatment options with your physician. Renuvion is typically used after liposuction, but treatment options are up to the discretion of the provider.
Description of After-Care Package: Depending on the number of areas being treated and your discussed anesthesia plan, Renuvion for Body Contouring recovery can be from a few days to a few weeks. Like any procedure, your treatment plan may result in a different level of recovery than someone else’s. Recovery expectations should always be discussed with your provider depending on your unique needs.
FREQUENTLY ASKED QUESTIONS
Renuvion is a single treatment. Results may be seen in as little as days or weeks and will continue to improve throughout the healing process.
Depending on the area of the body treated, the use of Renuvion only adds a few minutes per area to a standard liposuction case.
LATEST NEWS
AS SEEN IN











